Limited monkeypox vaccine supply would be stretched under FDA plan
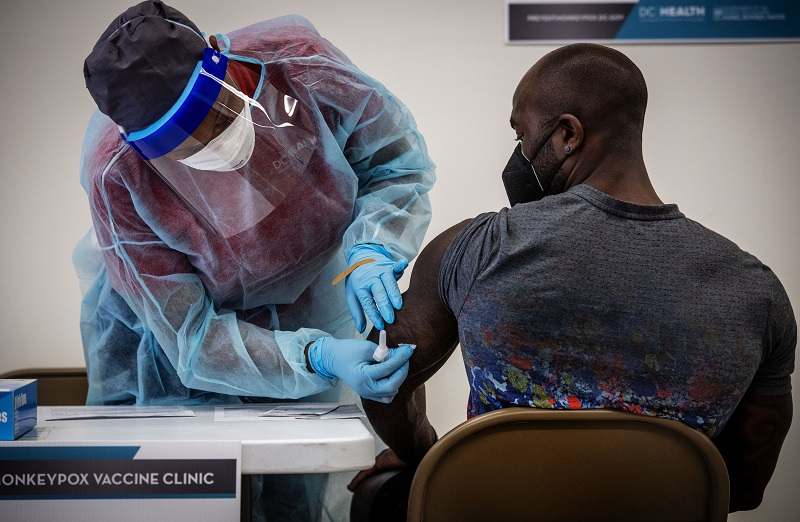
Registered nurse Uzo Okorie administers a shot to Kevin Carnell, a personal trainer, at a Washington, D.C., monkeypox vaccine clinic on June 28.
16:22 JST, August 9, 2022
WASHINGTON – Biden administration officials are set to announce Tuesday a new strategy to split monkeypox vaccine doses in hopes of vaccinating up to five times as many people against the virus, according to officials with direct knowledge of the plan.
The strategy, first described publicly by Food and Drug Administration Commissioner Robert Califf last week, would allow the Biden administration to stretch its limited supply of monkeypox vaccines by changing how those shots are administered. Rather than inject doses of Jynneos subcutaneously, a traditional way of delivering vaccines into the fatty tissue under the skin, the doses would instead be injected under the top layer of the skin. This approach, known as an intradermal injection, uses a thinner needle and less vaccine, but leads to a small bubble forming on the surface of the skin that can scar.
The change in injection method would maximize the immune reaction generated by the vaccine and allow U.S. officials to administer only one-fifth of the original dose, Califf told reporters last Thursday, stressing that the approach would not compromise safety or efficacy.
“It really means, basically, sticking the needle within the skin and creating a little pocket there into which the vaccine goes,” Califf said, comparing it to tests for tuberculosis and other injections performed by health-care workers. “This is really nothing highly unusual.”
But the planned change in vaccine dosing would be a large-scale, real-time experiment as officials race to stave off a monkeypox outbreak that has infected more than 8,900 people in the United States. If successful, the new vaccine plan would allow the Biden administration to transform hundreds of thousands of existing doses of Jynneos – the only FDA-approved vaccine for monkeypox – into several million potential shots. Although U.S. officials last month received nearly 800,000 additional doses of Jynneos, demand has rapidly outpaced supply and more doses are not expected for weeks.
The change is not without risk. People who receive the pared-down version of the Jynneos vaccine, which is intended as a two-dose regimen, may end up needing additional shots if the new vaccine strategy leads to an insufficient level of protection against the virus. Local officials may also struggle to administer the new strategy, which could require additional training and supplies such as new needles to safely deliver the shots.
“This isn’t a ‘snap your fingers and it’s done,'” said a public health expert who was briefed by the administration on its pending plan but was not authorized to comment. “There’s a lot of complexity to rolling this out right, many vaccination sites won’t be fully ready, and it’s not obvious that [the U.S. Department of Health and Human Services] has done everything it should to help get local preparations in place.”
HHS did not immediately respond to a request for comment. The FDA said Monday that the agency did not have any information to share.
Administration officials worked through the weekend on the logistics of the new strategy, drawing on prior studies into splitting vaccine doses, which is often referred to as “dose-sparing.” Infectious-disease expert Anthony Fauci and other senior administration officials have reviewed that data and support the planned change, according to two officials who spoke on the condition of anonymity because they were not authorized to comment.
The federal government must still take several steps before the vaccine change is legally permissible. Although Health and Human Services Secretary Xavier Becerra last Thursday declared monkeypox a public health emergency, he would need to issue a second declaration that would allow for “emergency use” of the existing monkeypox vaccines and other medical countermeasures, such as the plan to change how the shots are administered.
The Centers for Disease Control and Prevention is also working on guidance for health-care workers on how to administer the new vaccine strategy, according to two other people with knowledge of the planned announcement who spoke on the condition of anonymity because they were not authorized to comment.
Outside experts signaled their support for the idea. Daniel McQuillen, president of the Infectious Diseases Society of America, said in a statement that his organization “agrees with the strategy under consideration by FDA.”
The plan is “supported by compelling evidence of an equally strong immune response compared to the current strategy and could be an effective way to achieve the important goal of vaccinating more people,” McQuillen wrote.
But he noted that changing the way the shots would be administered could create new challenges, such as the greater risk of a skin reaction at the injection site. “For this strategy to succeed, strong public education about the way to correctly administer the vaccine intradermally and collection of data on its impacts will be essential.”
Scott Gottlieb, who served as commissioner of the FDA during the Trump administration, said officials were drawing on existing research into vaccines used for smallpox, a related virus.
“There’s a lot of data that FDA has looked at . . . [on] how we would extend doses of the smallpox vaccine. What they learned from those studies is transferrable to this vaccine for monkeypox,” Gottlieb said on CBS’ “Face the Nation” on Sunday.
The Biden administration has faced sustained criticism from patients, local public health officials and some lawmakers for not ordering more monkeypox vaccines earlier in the response, as demand now outpaces supply. Federal officials consider at least 1.6 million gay and bisexual men at highest risk for the virus and are urging them to get the shots.





