Health ministry to set new safety guidelines for animal organ, tissue use in humans
14:50 JST, August 25, 2022
The Health, Labor and Welfare Ministry will begin hammering out new guidelines for “xenotransplantation” — transplants of animal organs and tissues into humans — next fiscal year.
As research advances on creating organs for transplant that limit rejection by the immune system through new technology for modifying the donor animal’s genetics, xenotransplantations are now being conducted overseas, though only experimentally.
In light of such developments, the ministry aims to ensure the quality and safety of nonhuman organs to be transplanted into humans, with an eye to eventually conducting such transplants in the country.
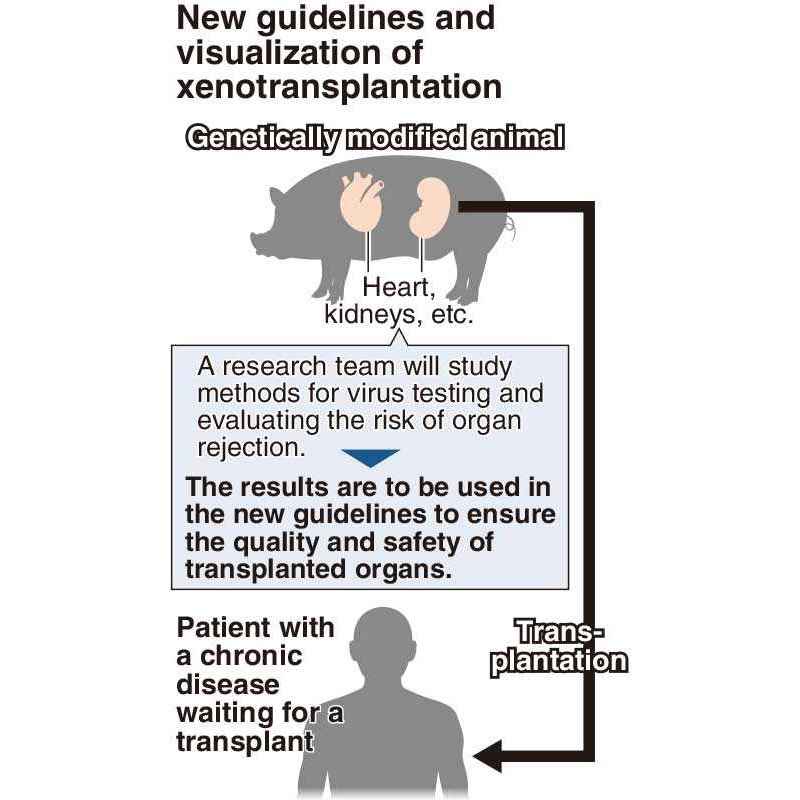
It is hoped that transplants of nonhuman organs and tissues from such sources as pigs will help solve a continuing shortage of organs needed by people suffering from chronic diseases.
However, transplant rejection, in which the transplanted organ is attacked by the immune system, presents a major challenge for xenotransplantation. It has also been pointed out that unknown viruses can stray into the cells of the organ to be transplanted.
The health ministry will establish a research team in the Japan Agency for Medical Research and Development to consider such matters as the quality of animal organs, such as those from pigs, and the risks of viral infection. The team will also study the latest technological trends and the likelihood of abnormalities occurring in humans if genetically modified, nonhuman organs and tissues are used for transplants.
Over a roughly three-year period, the team will compile a list of pointers for xenotransplantation that will include methods for virus testing and evaluating the risk of transplant rejection, as well as breeding requirements for animals to be used for xenotransplantation.
The ministry plans to include several tens of millions of yen for related outlays in its budgetary request for the next fiscal year.
In recent years, researchers have made strides in the development of nonhuman organs with low transplant rejection risk by making use of technologies such as genome editing, which allows them to make precise changes to targeted genes.
In the United States, doctors in January this year conducted the world’s first xenotransplantation of a pig heart into a human, using a heart from a pig with 10 genetic modifications to limit transplant rejection. (The patient died about two months later.)
In Japan, the health ministry drew up guidelines in 2016 under which transplants of pancreatic and other cells from animals into humans were approved on the condition that thorough follow-up studies would be conducted on the health of the cell recipients.
However, these guidelines were not produced with consideration for xenotransplantation of organs or tissues, and so far no such transplants have been conducted in Japan.




